Nobel laureate Otto Hahn is credited with the discovery of nuclear fission. Fission is likely one of the most vital discoveries of the twentieth century, but Hahn thought of one thing else to be his best scientific work.
In 1921, he was learning radioactivity on the Kaiser Wilhelm Institute for Chemistry in Berlin, Germany, when he observed one thing he couldn’t clarify. One of many parts he was working with wasn’t behaving because it should have. Hahn had unknowingly found the primary nuclear isomer, an atomic nucleus whose protons and neutrons are organized otherwise from the widespread type of the component, inflicting it to have uncommon properties. It took one other 15 years of discoveries in nuclear physics to have the ability to clarify Hahn’s observations.
We are two professors of nuclear physics who examine uncommon nuclei together with nuclear isomers.
The most typical place to search out isomers is inside stars, the place they play a task within the nuclear reactions that create new elements. Lately, researchers have begun to discover how isomers might be put to make use of for the advantage of humanity. They’re already used in medicine and will at some point supply highly effective choices for vitality storage in the form of nuclear batteries.
On the hunt for radioactive isotopes
Within the early 1900s, scientists had been on the hunt for brand new radioactive parts. A component is taken into account radioactive if it spontaneously releases particles in a course of referred to as radioactive decay. When this occurs, the component is remodeled over time into a distinct component.
At the moment, scientists relied on three standards to find and describe a brand new radioactive component. One was to have a look at chemical properties – how the brand new component reacts with different substances. In addition they measured the kind and vitality of the particles launched in the course of the radioactive decay. Lastly, they might measure how briskly a component decayed. Decay speeds are described utilizing the time period half-life, which is the period of time it takes for half of the preliminary radioactive component to decay into one thing else.
By the Twenties, physicists had found some radioactive substances with equivalent chemical properties however totally different half-lives. These are referred to as isotopes. Isotopes are totally different variations of the identical component which have the identical variety of protons of their nucleus, however totally different numbers of neutrons.
Uranium is a radioactive component with many isotopes, two of which happen naturally on Earth. These pure uranium isotopes decay into the component thorium, which in flip decays into protactinium, and every has its personal isotopes. Hahn and his colleague Lise Meitner had been the primary to find and determine many alternative isotopes originating from the decay of the component uranium.
All of the isotopes they studied behaved as anticipated, aside from one. This isotope appeared to have the identical properties as one of many others, however its half-life was longer. This made no sense, as Hahn and Meitner had positioned all of the identified isotopes of uranium in a neat classification, and there have been no empty areas to accommodate a brand new isotope. They referred to as this substance “uranium Z.”
The radioactive sign of uranium Z was about 500 times weaker than the radioactivity of the opposite isotopes within the pattern, so Hahn determined to verify his observations through the use of extra materials. He bought and chemically separated uranium from 220 kilos (100 kilograms) of extremely poisonous and uncommon uranium salt. The stunning results of this second, extra exact experiment advised that the mysterious uranium Z, now often known as protactinium-234, was an already identified isotope, however with a really totally different half-life. This was the primary case of an isotope with two totally different half-lives. Hahn revealed his discovery of the first nuclear isomer, though he couldn’t totally clarify it.
Neutrons full the story
On the time of Hahn’s experiments within the Twenties, scientists nonetheless considered atoms as a clump of protons surrounded by an equal variety of electrons. It wasn’t till 1932 that James Chadwick advised a 3rd particle – neutrons – had been additionally part of the nucleus.
With this new data, physicists had been instantly capable of clarify isotopes – they’re nuclei with the identical variety of protons and totally different numbers of neutrons. With this data, the scientific group lastly had the instruments to grasp uranium Z.
In 1936 Carl Friedrich von Weizsäcker proposed that two totally different substances may have the identical variety of protons and neutrons of their nuclei however in several preparations and with totally different half-lives. The association of protons and neutrons that leads to the bottom vitality is essentially the most secure materials and is named ‘floor state.’ Preparations leading to much less secure, larger energies of an isotope are referred to as isomeric states.
At first nuclear isomers had been helpful within the scientific group solely as a way to grasp how nuclei behave. However when you perceive the properties of an isomer, it’s doable to start out asking how they can be utilized.
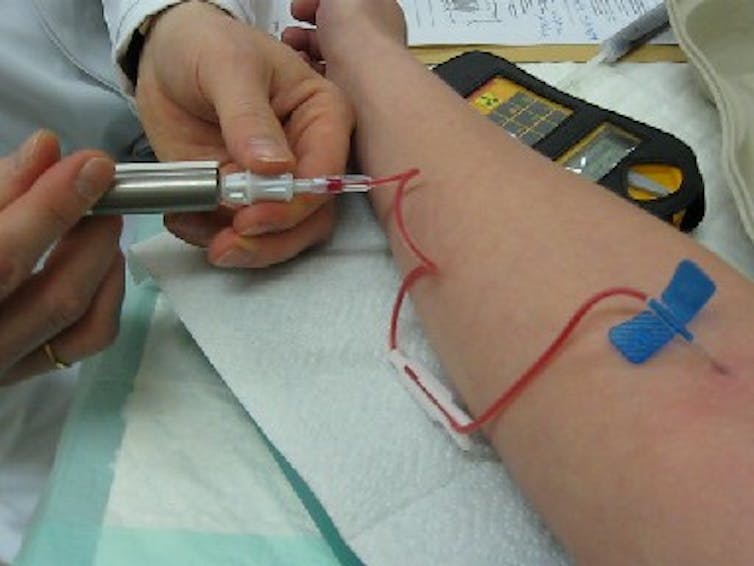
Isomers in medication and astronomy
Isomers have vital purposes in medication and are utilized in tens of hundreds of thousands of diagnostic procedures yearly. Since isomers endure radioactive decay, particular cameras can observe them as they transfer via the physique.
For instance, technetium-99m is an isomer of technetium-99. Because the isomer decays, it emits photons. Utilizing photon detectors, docs can observe how technetium-99m moves throughout the body and create images of the guts, mind, lungs, and different crucial organs to assist diagnose illnesses together with most cancers. Radioactive parts and isotopes are usually harmful as a result of they emit charged particles that harm bodily tissues. Isomers like technetium are safe for medical use as a result of they emit solely a single, innocent photon at a time and nothing else as they decay.
Isomers are additionally vital in astronomy and astrophysics. Stars are fueled by the vitality launched throughout nuclear reactions. Since isomers are present in stars, nuclear reactions are totally different than if a cloth had been in its floor state. This makes the examine of isomers crucial for understanding how stars produce all the weather within the universe.
Isomers sooner or later
A century after Hahn first found isomers, scientists are nonetheless discovering new isomers using powerful research facilities world wide, together with the Facility for Rare Isotope Beams at Michigan State College. This facility got here on-line in Could 2022, and we hope it’ll unlock greater than 1,000 new isotopes and isomers.
Scientists are additionally investigating whether or not nuclear isomers could possibly be used to build the world’s most accurate clock or whether or not isomers might at some point be the premise for the following technology of batteries. Greater than 100 years after the detection of a small anomaly in uranium salt, scientists are nonetheless on the hunt for brand new isomers and have simply begun to disclose the complete potential of those fascinating items of physics.![]()
This text by Artemis Spyrou, Professor of Nuclear Physics, Michigan State University, and Dennis Mücher, Affiliate Professor of Nuclear Physics, University of Guelph is republished from The Conversation beneath a Artistic Commons license. Learn the original article.


